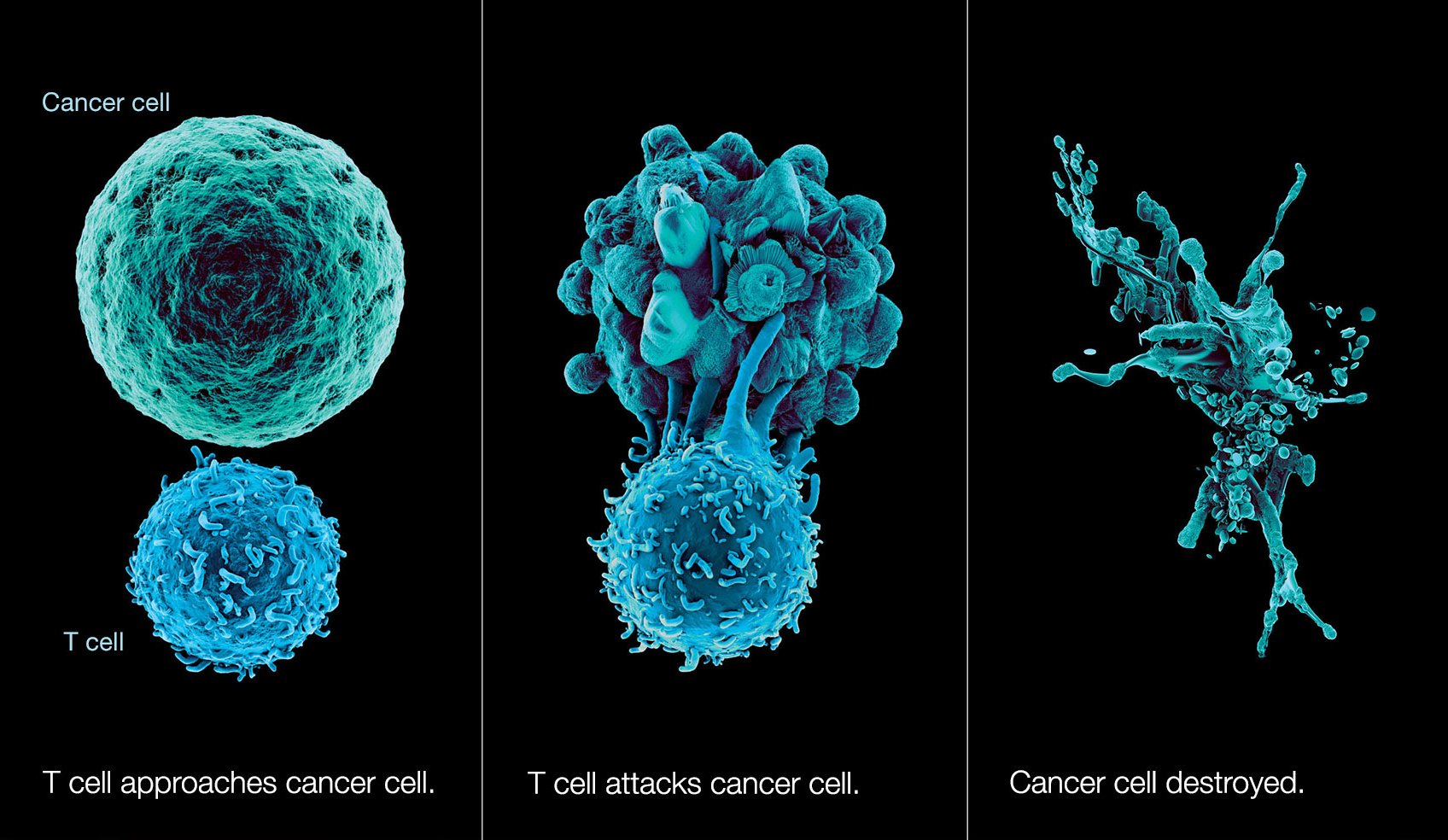
One of the human bodys greatest features is its natural antivirus protection. If your immune system is working normally, it produces legions of T-cells that go around looking for abnormalities like cancer cells just to gang up and destroy them. They do this by grabbing on to little protein fragments called antigens that live on the surface of the bad cells and tattle on their whereabouts to the immune system. Once the T-cells have a stranglehold on these antigens, they can release toxins that destroy the bad cell, while minimizing collateral damage to healthy cells.
This rather neat human trick doesnt always work, however. Cancer cells sometimes mask themselves as healthy cells, or they otherwise thwart T-cell attacks by growing so many antigens on their surface that the T-cells have no place to grab onto.
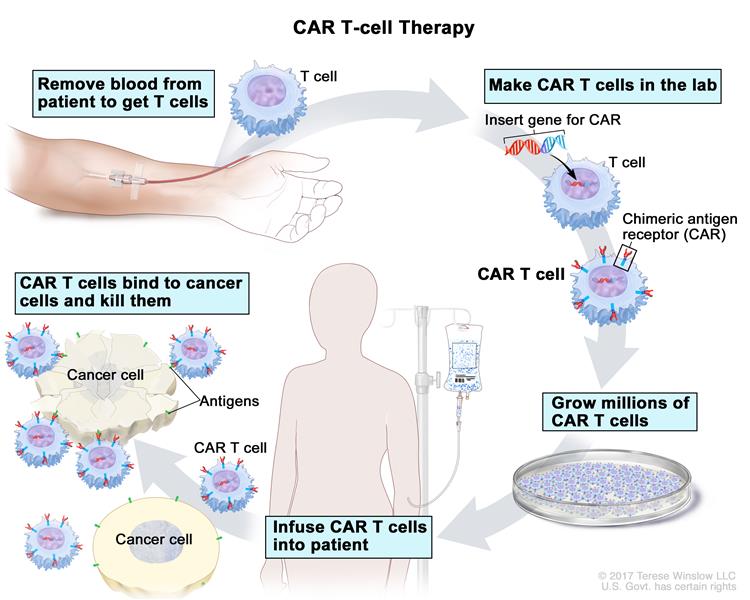
Medical science has come up with a fairly new method of outfoxing these crafty cancer cells called CAR T-cell therapy. Basically, they withdraw blood from the patient, extract the T-cells, and replace the blood. The T-cells are sent off to a CRISPR lab, where they get injected with a modified, inactive virus that introduces a new gene which causes the T-cells to sprout a little hook on their surface.
This hook, which theyve dubbed the chimeric antigen receptor (CAR), allows the T-cell to chemically see through the cancer cells various disguises and attack them. The lab multiplies these super soldiers and sends them back to the treatment facility, where they are injected into the patients front lines.
Protein is Key to Unlocking the CARs Potential
Currently, T-cell therapy only seems to work in blood-based cancers like leukemia, and is ineffective at fighting solid tumor-producing cancers. But a team at Cardiff University has discovered a new T-cell that could change the game. This T-cell interacts with a certain protein called MR1 that appears on the surface of every cell in the body. When it analyzes the MR1 proteins of cancer cells, it can tell that the metabolism going on inside the cell is distorted, and reports this miscreant cell back to the immune system.
This discovery is still in its infancy, but it has great potential. Editing T-cells to interact with MR1 proteins specifically could one day kill a wide range of cancer cells in human patients. For now, the trials are limited to the lab, but the outlook is good: researchers have succeeded in destroying 10+ types of cancer, including many solid-tumor types.
CAR Is Still a Bit Rickety
CAR T-cell therapy is still quite an expensive, bleeding-edge cancer weapon, but its far less ravaging to the body than chemotherapy, though low doses are sometimes necessary to make room for the gene-edited cells. And there are still some kinks to be worked out patients can have serious side effects, some of which cause neurological difficulties like confusion, and trouble with speaking and understanding language.
Another issue is the timeline for the CAR T-cell therapy process it takes 2-3 weeks to get from initial blood withdrawal to injecting the personalized dose back into the patient. In June of 2019, trials began with an off-the-shelf version of CAR T-cells made from healthy cell donors and stored. The downside to this off-the-shelf solution is that the donors cells may react poorly with the recipients, but not always. All in all, this seems like progress to us.
Main image photo by @nci on Unsplash
